Our Science
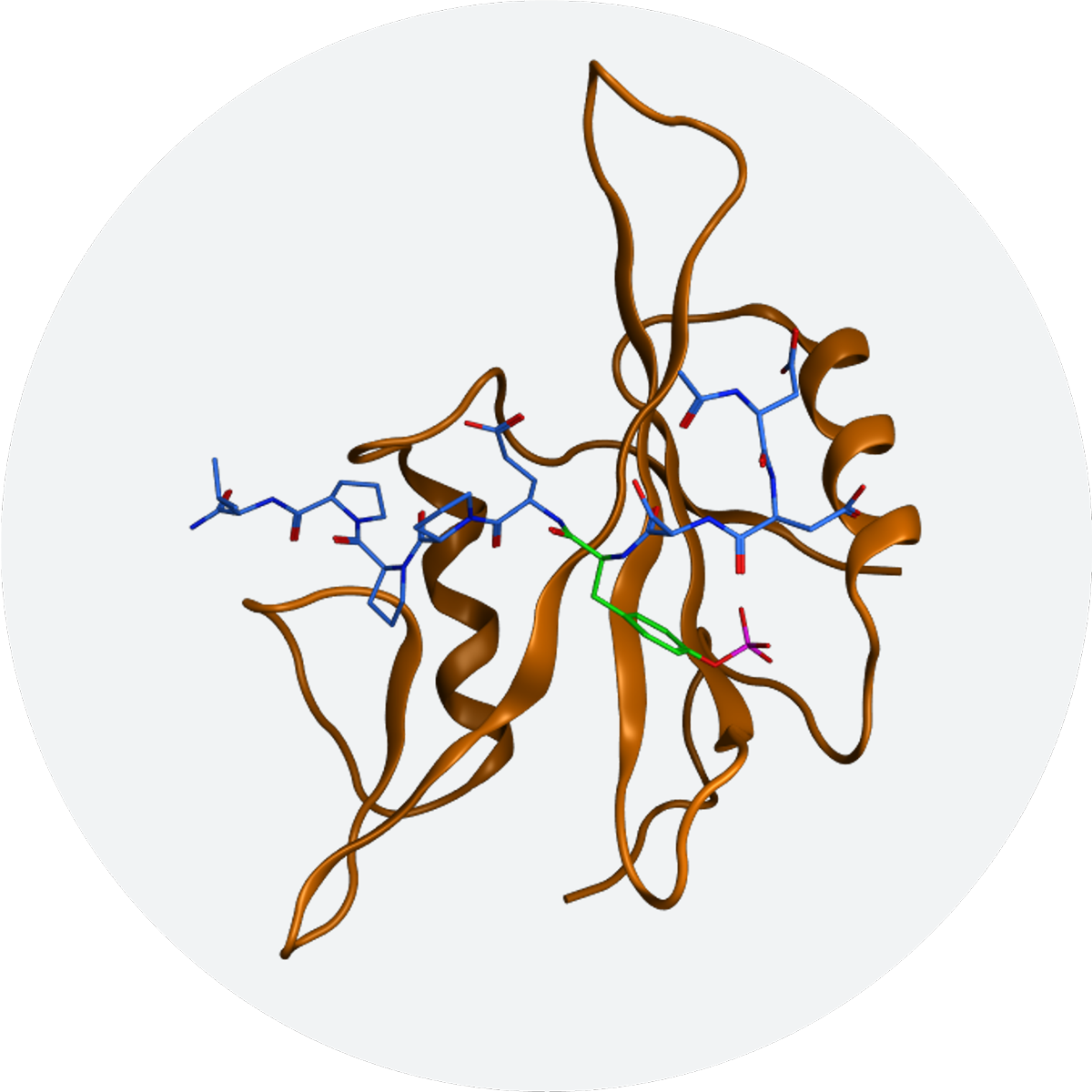
SH2 domains are critical for regulating the location and activity of proteins and play a key role in cellular signal transduction that, when abnormal, drives certain inflammatory disorders and cancers. They are small modules found in many proteins that mediate protein-protein interactions by binding to phospho-tyrosine containing motifs. There are 120 SH2 domains identified in humans (Molecular Cell, vol. 22, p.851-868).
Discovered over 30 years ago, SH2 domains have been widely recognized as attractive drug targets in many disease pathways. However, until recently, efforts to develop drug-like compounds to inhibit their function have been unsuccessful, and SH2 domains therefore have been considered undruggable.
Recludix’s SH2-focused platform integrates new chemical approaches and technologies to overcome this challenge, enabling the successful development of potent, selective and drug-like SH2 domain inhibitors.
There are numerous proteins implicated in disease pathology that have SH2 domains. Recludix’s initial programs are focused on Signal Transducer and Activator of Transcription (STAT) proteins found within the Janus Kinase (JAK)/STAT signaling pathway, as well as Bruton’s tyrosine kinase (BTK) protein.
Targeting STAT Proteins
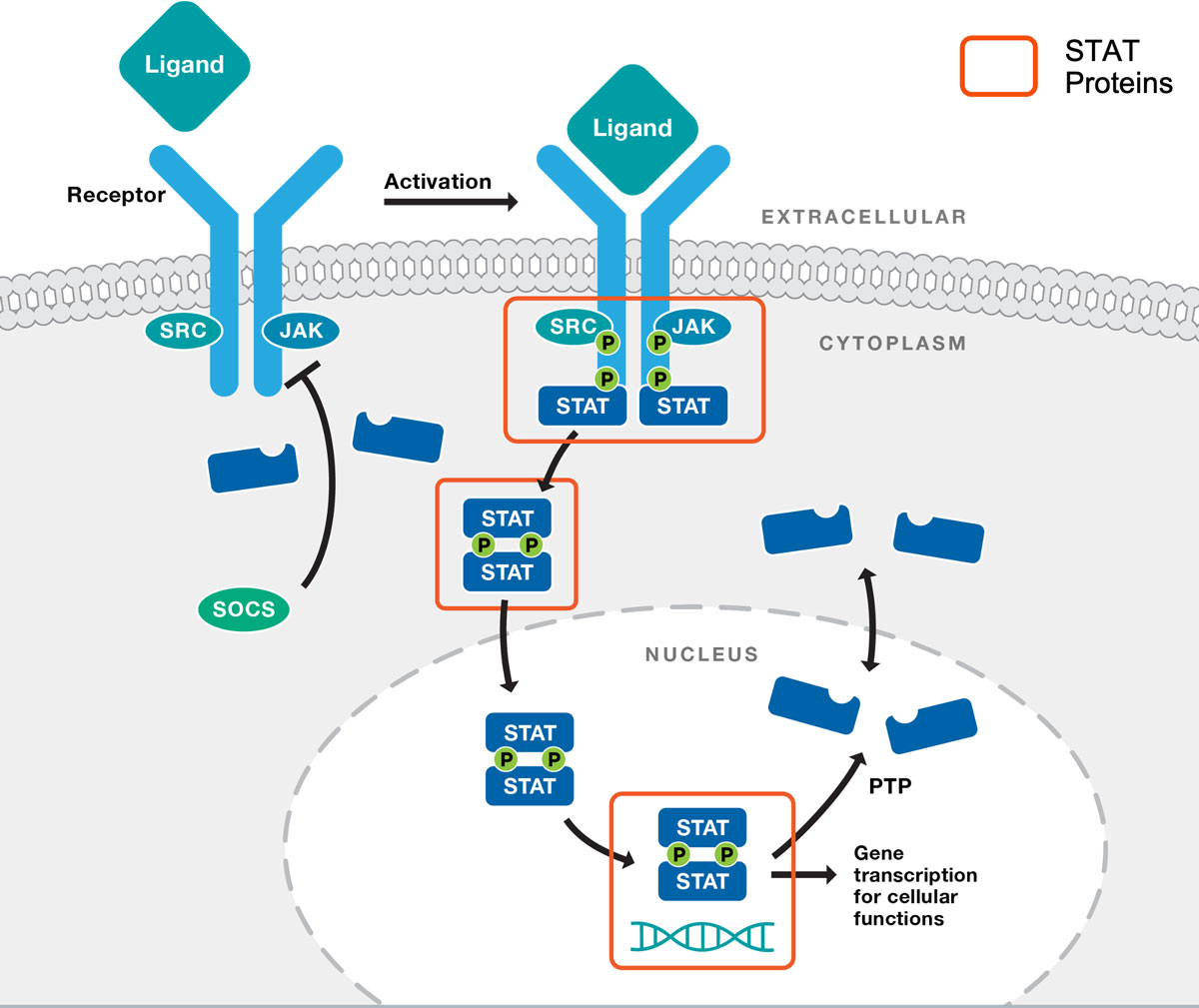
JAK/STAT signaling pathways play a privileged role in the immune system, regulating the growth, differentiation and activity of many types of immune cells. JAK/STAT pathways are abnormally activated in many inflammatory diseases as well as many cancers of the immune system. Targeting JAK/STAT pathways to treat inflammatory diseases and cancer has been clinically validated as an effective strategy by the success of JAK and TYK2 kinase inhibitors.
STAT proteins are found downstream in the JAK/STAT pathway, and therefore selective STAT inhibitors are predicted to be more targeted agents with fewer side effects. STAT proteins are both signaling proteins and transcription factors. They are phosphorylated, and thereby activated, by JAK family kinases in response to cytokine stimulation. Upon activation, STAT proteins dimerize and translocate to the nucleus, where they activate transcriptional programs that drive cell growth, differentiation and function. Each of the seven STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, STAT6) contain an SH2 domain that mediates the two activities required for STAT proteins to act as transcription factors – their activation by cytokine stimulation and their dimerization. The inhibition of SH2 domains therefore interferes with STAT protein activity at multiple levels and provides an efficient and selective means to inhibit JAK/STAT signaling.
Targeting BTK
BTK is a signaling protein that is part of the tyrosine-protein kinase (TEC) family and plays an important role in the function and activation of B cells and myeloid cells.
BTK inhibitors that are approved to treat B-cell malignancies target the catalytic tyrosine kinase domain. Because other proteins’ kinase domains share close homology to BTK, it has been challenging to develop a highly specific BTK kinase inhibitor.
Recludix’s approach to drugging BTK is through inhibiting the SH2 domain, not the kinase domain, which enables a higher degree of specificity. Additionally, by blocking the SH2 domain, Recludix compounds impact multiple pro-inflammatory pathways of BTK — blocking access to key kinase targets and other pathways mediated by the scaffolding function.
