STAT3
STAT3 in Inflammatory Disease
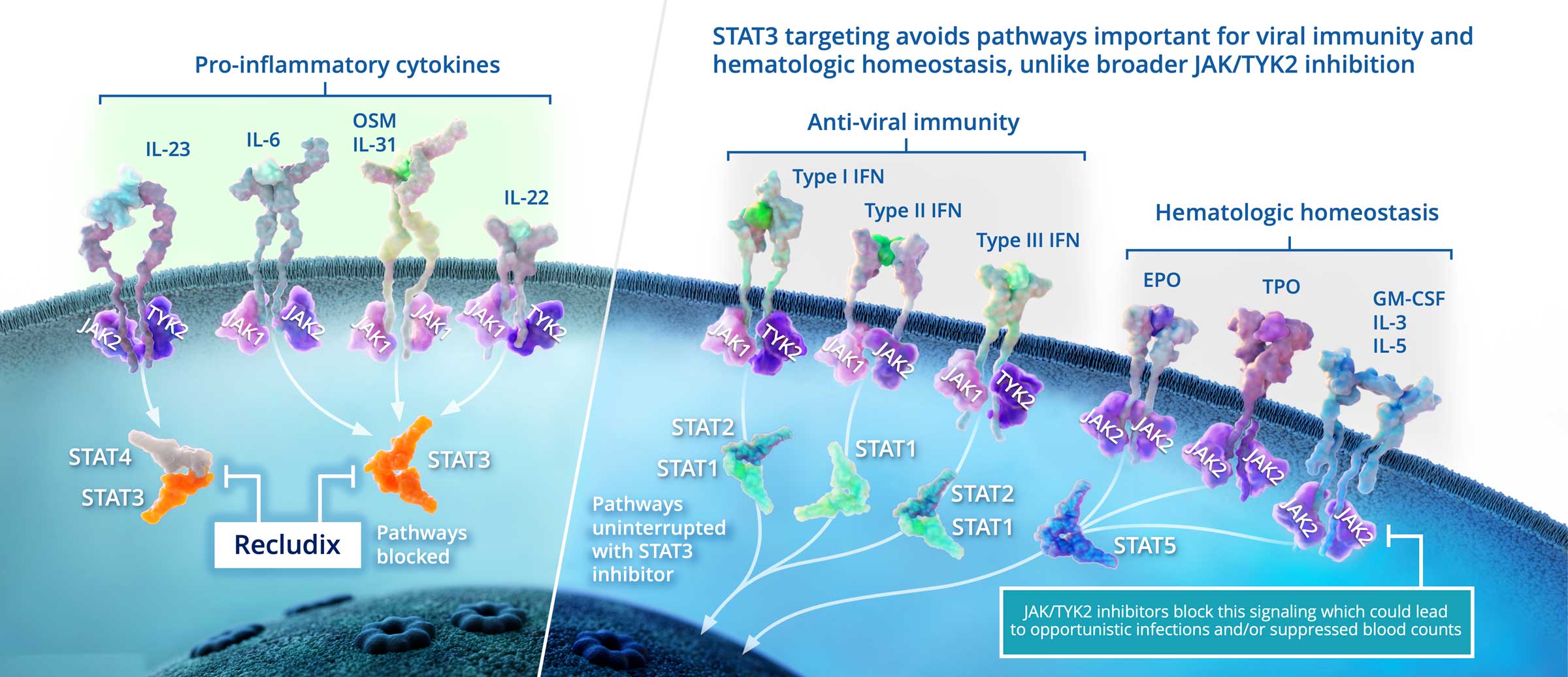
Targeted STAT3 inhibition with an oral, reversible, small molecule drug represents an attractive alternative to biologics and to Janus Kinase (JAK) family inhibitors, including TYK2 inhibitors, to inhibit Th17 cell activity and treat Th17-driven inflammatory and autoimmune diseases. Importantly, STAT3 inhibition offers potential efficacy beyond Th17 / IL-17 driven diseases in clinical indications where blocking the IL-6 pathway is clinically validated.
The interleukin cytokines IL-23 and IL-6 signal through STAT3 and promote the generation and function of pathogenic T helper type-17 (Th17) cells, a type of immune cell that is pro-inflammatory. They are considered pivotal players in certain inflammatory diseases, including rheumatoid arthritis, psoriasis, inflammatory bowel disease and others. Monoclonal antibody therapies that target individual cytokine mechanisms have been approved as treatments; however, these medicines require injection, are often restricted to subsets of patients, and are slow to clear from the body if treatment discontinuation is required.
Oral, reversible therapies inhibiting the JAK family, including TYK2, have demonstrated clinical efficacy in Th17-driven diseases. However, JAK family inhibition can be associated with changes in immune surveillance and hematopoiesis, leading to dose limiting safety concerns. Recludix’s therapeutic approach targeting STAT3 is expected to be safer in this regard, as cytokines critical to anti-viral immunity and hematologic homeostasis do not utilize STAT3 signaling.
STAT3 is a First and Best-In-Class Opportunity to Inhibit Clinically Validated Inflammatory Disease Pathways